
Our research program spans several areas of organic chemistry, including chemistry of nitroxy compounds and N-heterocycles, natural product synthesis, catalysis, organoboron chemistry, and organic photochemistry. The unifying theme of our research is the development of simple and efficient synthetic methods that streamline access to complex organic molecules. Recent examples of our work include:
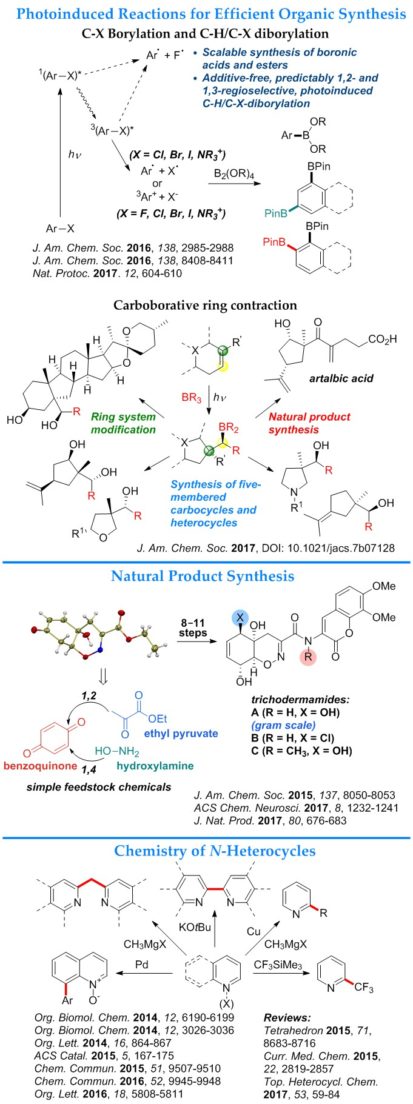
- Photoinduced carboborative ring contraction. The reaction enables a stereo- and regioselective access to five-membered carbocycles and heterocycles.
- Photoinduced C–X (X = F, Cl, Br, I, NR3+) borylation. The reaction produces boronic acids and esters in the absence of catalysts and additives and without rigorous exclusion of air and moisture.
- Photoinduced regioselective dual C–H/C–X diborylation. The reaction produces 1,2- and 1,3-diboronic esters from haloarenes. The regioselectivity of the reaction is determined by the solvent and the substituents in the aromatic ring of the haloarene.
- Total synthesis of trichodermamides A–C. The concise synthesis has enabled a study of the mechanism of action of the cytotoxic trichodermamide B, and produced simplified structural analogues of trichodermamides that inhibit the Amyloid β – CD36 interaction implicated in pathogenesis of Alzheimer’s disease.
Topics of Interest
- Photoinduced reactions for efficient organic synthesis
- Organoboron chemistry
- Chemistry of heterocyclic N-oxides
- Synthesis of biologically active natural products
- Enantioselective catalysis
