We employ a range of experimental techniques, including kinetic (for example, Variable Time Normalization Analysis) and spectroscopic (EPR, DOSY NMR, UV/Vis absorption and emission spectroscopy) to investigate mechanisms of reactions developed in our lab or in collaboration with other researchers. We augment experimental studies with detailed computational investigations, using a variety of computational techniques (for example, DFT and TD-DFT calculations, distortion/interaction activation strain model analysis, and analysis of non-covalent interactions) to gain insight into various reaction pathways, the nature of reactive intermediates, and the inter- and intramolecular interactions underpinning the experimentally observed reactivity and selectivity.
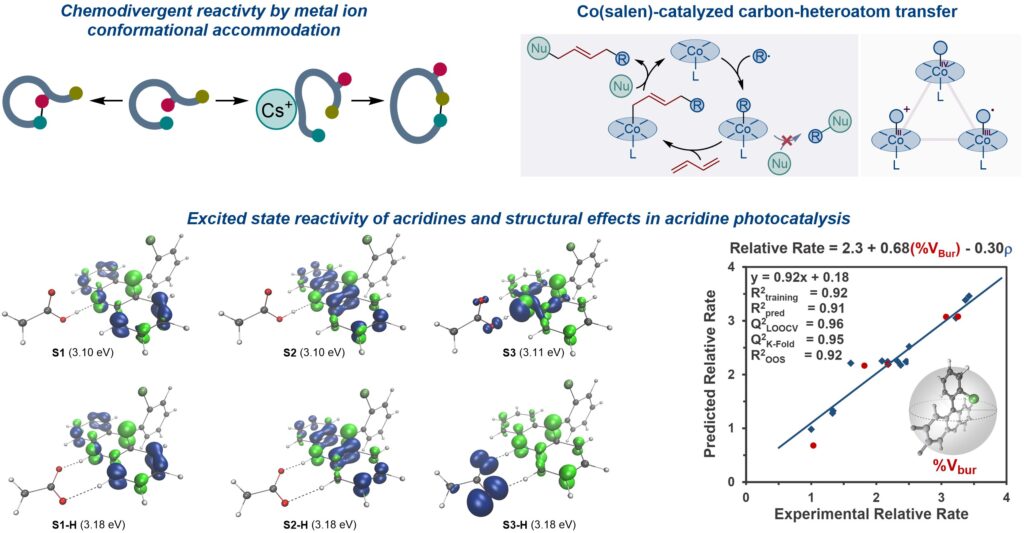
Our studies of divergent chemoselectivity of oxadiaza excision reactions uncovered a new role that cesium ions can have in modulating organic reactions. The divergent chemoselectivity was due to metal-specific conformational preferences of cyclization transition states. These studies indicate that differences in conformational accommodation of specifically selected chelated metal ions can be harnessed to produce structurally different products and access broader chemical space from the same precursors.
Our studies of the mechanism of cobalt-catalyzed 1,4-carboamination highlighted the roles of formal high oxidation state cobalt species in promoting radical–polar crossover reactions. Studies pointed to the involvement of formal Co(IV) species, while effective oxidation state analysis revealed the inverted ligand field character of the species.
We also studied the mechanism of acridine photocatalysis, leveraging a range of experimental and computational methods. Our studies revealed that the reactions with carboxylic acids proceed by proton-coupled electron transfer in the singlet excited state. We also showed that acridine can promote hydrogen atom transfer from Si–H bonds in the triplet excited state.
Larionov Group | All Rights Reserved.