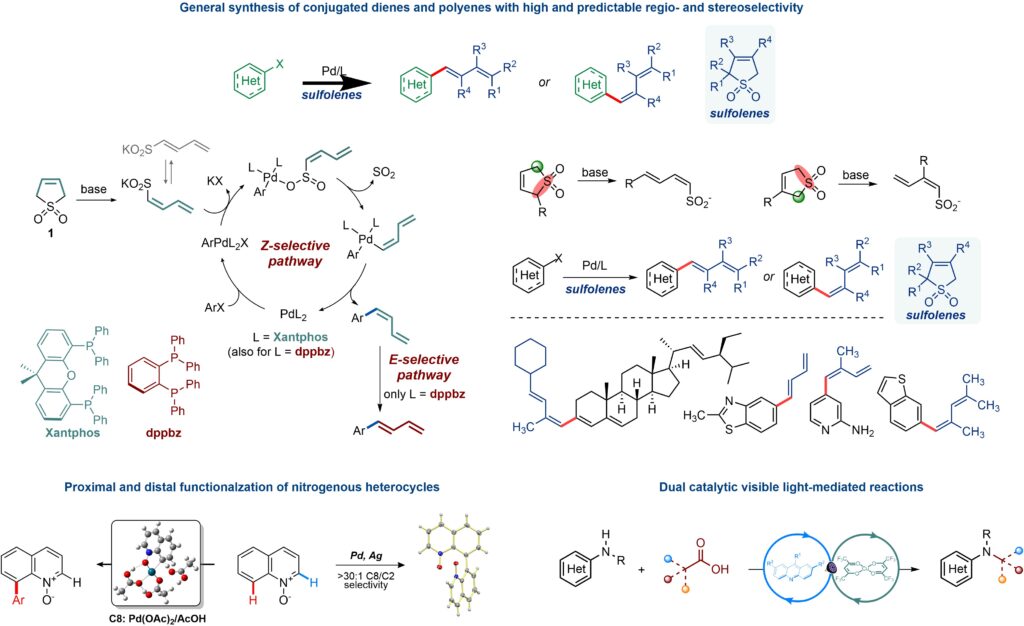
We develop transition metal catalyzed reactions that facilitate construction of medicinally and synthetically important molecular fragments. For example, we developed a catalytic approach for a regio- and stereoselective introduction of substituted diene moieties. The catalytic dienylation provides a one-step shortcut for the synthesis of dienes and polyenes, using sulfolenes as dienylation reagents. The reaction is stereodivergent: both E- and Z-selective dienylation is possible, and the configurations of the double bonds in the diene units are determined by the phosphine ligand and the sulfolene precursor.
We also developed a range of methods for selective C–H and C–X functionalization of nitrogen heterocycles. The reactions enable regioselective functionalization of proximal and distal positions in N-heterocycles.
Another area of interest is the development of dual catalytic reactions that merge visible light mediated generation of open shell species with transition metal-catalyzed reactions. The synthetic methods that have emerged from these studies enable various carbon–heteroatom bond-forming reactions and provide chemoselective segways to medicinally and synthetically valuable molecular targets.
Larionov Group | All Rights Reserved.